Blog
Molar Mass of Sodium Hydroxide Explained: A Comprehensive Guide
Molar Mass of Sodium Hydroxide: A Comprehensive Guide
The molar mass of sodium hydroxide is a key concept in chemistry that everyone in the field needs to grasp. Sodium hydroxide, often abbreviated as NaOH, is a strong base used widely in both industries and laboratories. By knowing its molar mass, you can accurately prepare solutions and conduct chemical reactions. This article explores the concept of molar mass, demonstrates how to calculate it for sodium hydroxide, and explains why it’s crucial in various chemical processes.
What is Molar Mass?
To understand the molar mass of sodium hydroxide, you first need to know what “molar mass” means. Molar mass represents the mass of one mole of a substance, measured in grams per mole (g/mol). It reflects the sum of the atomic masses of all atoms in a molecule. For sodium hydroxide, you add up the atomic masses of sodium (Na), oxygen (O), and hydrogen (H).
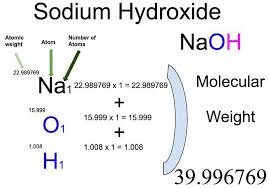
How to Calculate the Molar Mass of Sodium Hydroxide
Follow these steps to calculate the molar mass of sodium hydroxide:
- Identify the Atomic Masses:
- Sodium (Na) has an atomic mass of approximately 22.99 g/mol.
- Oxygen (O) has an atomic mass of about 16.00 g/mol.
- Hydrogen (H) has an atomic mass of roughly 1.01 g/mol.
- Add the Atomic Masses:
- Sodium hydroxide has the formula NaOH, consisting of one sodium atom, one oxygen atom, and one hydrogen atom.
- To calculate the molar mass of NaOH: Molar Mass of NaOH=22.99 (Na)+16.00 (O)+1.01 (H)=40.00 g/mol\text{Molar Mass of NaOH} = 22.99 \text{ (Na)} + 16.00 \text{ (O)} + 1.01 \text{ (H)} = 40.00 \text{ g/mol}Molar Mass of NaOH=22.99 (Na)+16.00 (O)+1.01 (H)=40.00 g/mol
Why Knowing the Molar Mass of Sodium Hydroxide Matters
Understanding the molar mass of sodium hydroxide is essential for several reasons:
- Solution Preparation: Accurate molar mass calculations ensure you prepare sodium hydroxide solutions precisely, which is vital for laboratory and industrial applications.
- Chemical Reactions: Use the molar mass to determine the amounts of reactants and products in chemical reactions, ensuring they proceed correctly.
- Safety and Handling: Knowing the molar mass helps you understand the quantities needed and handle sodium hydroxide safely, minimizing the risk of overuse or underuse.
Practical Uses of Sodium Hydroxide

With a molar mass of 40.00 g/mol, sodium hydroxide finds various practical applications:
- Industrial Processes: Manufacturers use sodium hydroxide in processes such as soap production, paper pulping, and textile processing.
- Laboratory Experiments: Labs use sodium hydroxide for titrations and as a standard solution in various chemical analyses.
- Cleaning Agents: Many cleaning products include sodium hydroxide for its ability to dissolve fats and oils.
Conclusion
Understanding the molecular weight of sodium hydroxide is essential for anyone involved in chemistry. Accurate knowledge of this value, 40.00 g/mol, allows you to prepare solutions with precision, conduct chemical reactions effectively, and ensure safe handling of sodium hydroxide. Whether you’re working in a laboratory or an industrial setting, mastering the calculation of molar mass can significantly impact your work’s accuracy and efficiency.
Frequently Asked Questions
How do I find the molar mass of NaOH?
To find the molecular weight of sodium hydroxide (NaOH), you need to sum the atomic masses of its constituent elements:
- Identify the Atomic Masses:
- Sodium (Na): approximately 22.99 g/mol
- Oxygen (O): approximately 16.00 g/mol
- Hydrogen (H): approximately 1.01 g/mol
- Add the Atomic Masses:Molar Mass of NaOH=22.99 (Na)+16.00 (O)+1.01 (H)=40.00 g/mol\text{Molar Mass of NaOH} = 22.99 \text{ (Na)} + 16.00 \text{ (O)} + 1.01 \text{ (H)} = 40.00 \text{ g/mol}Molar Mass of NaOH=22.99 (Na)+16.00 (O)+1.01 (H)=40.00 g/mol
This total represents the mass of one mole of sodium hydroxide.
Why is the molar mass of NaOH 40?
The molecular weight of sodium hydroxide (NaOH) is 40.00 g/mol because it is the sum of the atomic masses of its three constituent atoms:
- Sodium (Na) has an atomic mass of about 22.99 g/mol.
- Oxygen (O) has an atomic mass of about 16.00 g/mol.
- Hydrogen (H) has an atomic mass of about 1.01 g/mol.
When you add these values together (22.99 + 16.00 + 1.01), you get 40.00 g/mol. This value represents the mass of one mole of sodium hydroxide.
What is 1 molar of NaOH?
1 molar (1 M) of sodium hydroxide (NaOH) refers to a solution that contains 1 mole of NaOH dissolved in 1 liter of solvent (usually water). It is a measure of concentration used in chemistry to describe how much of the substance is present in a given volume of solution.
How to find the mol of NaOH?
To find the number of moles of sodium hydroxide (NaOH), you can use the following formula:Moles=Mass (g)molecular weight (g/mol)\text{Moles} = \frac{\text{Mass (g)}}{\text{Molar Mass (g/mol)}}Moles=Molar Mass (g/mol)Mass (g)
For example, if you have 20 grams of NaOH and you know its molar mass is 40.00 g/mol:Moles of NaOH=20 g40.00 g/mol=0.5 moles\text{Moles of NaOH} = \frac{20 \text{ g}}{40.00 \text{ g/mol}} = 0.5 \text{ moles}Moles of NaOH=40.00 g/mol20 g=0.5 moles
This calculation allows you to

