Blog
Sodium Hydroxide Chemical Formula: Understanding Its Uses
Sodium hydroxide chemical formula, also known as NaOH, is a vital component in various industrial and household applications. Commonly referred to as lye or caustic soda, sodium hydroxide is a powerful alkali that plays a crucial role in different manufacturing processes, from cleaning to soap production. In this article, we’ll explore the sodium hydroxide chemical formula, its applications, and essential safety precautions.
What Is Sodium Hydroxide?
Sodium hydroxide (NaOH) is a chemical compound made of sodium (Na), oxygen (O), and hydrogen (H). This simple combination gives sodium hydroxide its chemical formula NaOH, making it a highly reactive and versatile substance. As a strong base, sodium hydroxide is used in a wide range of industries due to its ability to break down organic materials and neutralize acids.
Understanding the Sodium Hydroxide Chemical Formula
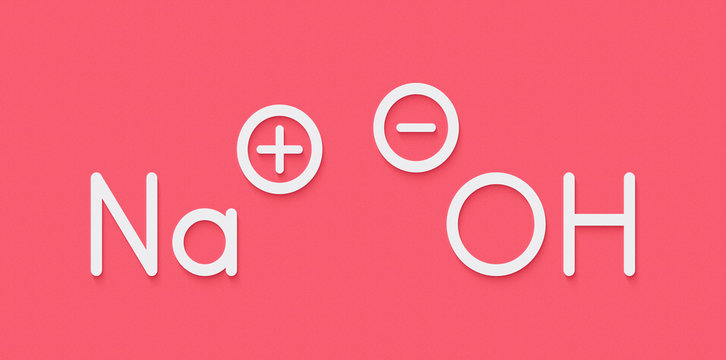
The sodium hydroxide chemical formula, NaOH, consists of one sodium atom (Na), one oxygen atom (O), and one hydrogen atom (H). Here’s a closer look at its structure:
- Na (Sodium): A soft, silver-colored metal that reacts with water and air.
- O (Oxygen): A non-metallic element that combines with hydrogen to form hydroxide.
- H (Hydrogen): The lightest and most abundant chemical element.
The combination of these three elements creates a potent base that can dissolve in water, generating heat and forming an alkaline solution. This solution is often used to adjust pH levels or as a cleaning agent in industries and households.
Applications of Sodium Hydroxide
Sodium hydroxide has numerous applications due to its strong alkaline properties. Let’s dive into the major uses of the sodium hydroxide chemical formula:
1. Manufacturing of Soap and Detergents
The sodium hydroxide chemical formula plays a key role in saponification, the process of making soap. When combined with fats or oils, sodium hydroxide reacts to form soap, breaking down grease and grime effectively. This process is essential for creating both bar soaps and liquid detergents.
2. Paper and Pulp Industry
The paper industry heavily relies on sodium hydroxide to process wood into pulp. The sodium hydroxide chemical formula helps break down lignin, a component of wood, allowing the extraction of cellulose fibers used in paper production.
3. Textile Industry
In textiles, sodium hydroxide is used in the dyeing and bleaching of fabrics. It helps soften and process raw materials like cotton, enhancing their absorbency and durability during the manufacturing process.
4. Food Processing
The food industry uses sodium hydroxide to peel fruits and vegetables, process cocoa, and make food additives. Although the sodium hydroxide chemical formula may sound harsh, it’s regulated for food safety and used in small amounts to ensure product quality.
5. Water Treatment
In water treatment plants, sodium hydroxide is utilized to adjust pH levels and neutralize acidic contaminants. It also helps in removing heavy metals from water, making it safer for consumption and environmental discharge.
6. Cleaning Agents
The sodium hydroxide chemical formula is commonly found in household cleaning products. As a powerful degreaser, it is effective in unclogging drains, cleaning ovens, and removing tough stains. However, due to its corrosive nature, users must handle it with care.
Safety Precautions When Handling Sodium Hydroxide
Although sodium hydroxide is a useful compound, it can be hazardous if not handled properly. Here are some important safety tips for using products with the sodium hydroxide chemical formula:
- Wear Protective Gear: Always wear gloves, goggles, and protective clothing when working with sodium hydroxide. It can cause burns if it comes into contact with the skin or eyes.
- Ensure Proper Ventilation: Sodium hydroxide can release fumes that are harmful when inhaled. Use it in well-ventilated areas or with proper exhaust systems.
- Avoid Ingestion: Sodium hydroxide is toxic if swallowed. Keep it out of reach of children and pets, and never store it in containers that may be mistaken for food or drinks.
- Neutralize Spills: In case of a sodium hydroxide spill, neutralize it with a mild acid like vinegar before cleaning up.
- Store Safely: Keep sodium hydroxide in a dry, cool place, and ensure the container is sealed tightly to prevent moisture absorption.
Environmental Impact of Sodium Hydroxide
Sodium hydroxide is generally safe for the environment when handled correctly, but it can pose risks if released in large amounts. The sodium hydroxide chemical formula, when mixed with water, increases the pH, making water more alkaline. This can harm aquatic life if not neutralized properly before disposal. Industries using sodium hydroxide must comply with environmental regulations to minimize its impact on ecosystems.
How to Safely Dispose of Sodium Hydroxide
Proper disposal of sodium hydroxide is essential to prevent environmental harm. Here’s how to do it safely:
- Dilute with Water: Diluting sodium hydroxide with a large amount of water can neutralize its strength, making it safer for disposal.
- Contact Local Waste Management: For large quantities, contact your local hazardous waste disposal service for guidance on proper disposal procedures.
- Neutralize: Before disposing of sodium hydroxide down the drain, neutralize it with a mild acid, such as vinegar or citric acid.
Conclusion
The sodium chemical formula, NaOH, is more than just a chemical—it’s an essential component in industries like soap making, water treatment, food processing, and more. Despite its usefulness, safety precautions must always be taken when handling sodium hydroxide to avoid potential hazards.
