Blog
Formula for Phosphoric Acid: A Comprehensive Guide
Phosphoric acid is one of the most widely used acids in various industries, and its formula is a fundamental aspect of chemistry. The formula for phosphoric acid is H₃PO₄. Understanding this formula is crucial because phosphoric acid has numerous applications, from agriculture to food processing.
In this article, we’ll explore the formula for phosphoric acid, break down its structure, discuss its key uses, and provide practical insights. Whether you’re a student or a professional, this guide will offer all the necessary details.
What is Phosphoric Acid?
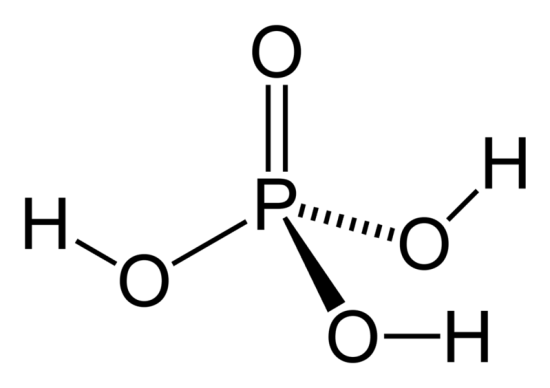
Phosphoric acid is an inorganic acid commonly known as orthophosphoric acid. Its chemical formula, H₃PO₄, reflects that it contains three hydrogen (H) atoms, one phosphorus (P) atom, and four oxygen (O) atoms.
Structure of the Formula for Phosphoric Acid
The formula for phosphoric acid, H₃PO₄, represents its molecular structure. Here’s a breakdown:
- H₃: This shows that there are three hydrogen atoms attached.
- P: This single phosphorus atom forms the backbone of the acid.
- O₄: Four oxygen atoms are connected to the phosphorus atom.
This structure makes phosphoric acid a triprotic acid, meaning it can donate three protons (H⁺ ions) when dissolved in water.
Importance of the Formula for Phosphoric Acid
The formula for phosphoric acid is not just a representation of its molecular makeup but also plays a critical role in its applications. Understanding the components of H₃PO₄ is essential for professionals working in industries like:
- Agriculture: Phosphoric acid is a key component in fertilizers.
- Food Processing: It’s used to acidify foods and beverages.
- Pharmaceuticals: Acts as an ingredient in medications.
- Metal Treatment: Used to clean and rustproof metals.
By understanding the formula, industries can apply phosphoric acid in a safe and effective manner.
Applications of Phosphoric Acid in Industries
1. Agriculture: Fertilizers and the Role of H₃PO₄
The formula for phosphoric is essential in the production of fertilizers. These fertilizers improve soil fertility and help crops thrive, which is why phosphoric acid plays a crucial role in global agriculture.
2. Food and Beverage Industry: Acidity Regulation
In the food industry, manufacturers use phosphoric acid, derived from H₃PO₄, to acidify soft drinks and other processed foods. It gives beverages their tangy taste while acting as a preservative. It’s a common ingredient in soda and other carbonated drinks, where maintaining the right pH level is important for both taste and safety.
3. Pharmaceuticals: Purity and Quality
Phosphoric acid also plays a significant role in pharmaceuticals. Its formula makes it a suitable ingredient in medicines, dental products, and cleaning solutions. It can be found in over-the-counter medications for digestive relief or as an additive to enhance stability.
4. Metal Treatment and Surface Preparation
The formula for phosphoric allows it to react effectively with metals. Its use helps ensure that metals are free from corrosion, improving their longevity and durability.
How to Use the Formula for Phosphoric Acid in Calculations
The formula H₃PO₄ is essential in calculating concentrations, reactions, and the molecular weight of phosphoric. For example, to determine the molar mass of phosphoric acid, you would add up the atomic weights of its components:
- Hydrogen (H): 1.008 g/mol × 3 = 3.024 g/mol
- Phosphorus (P): 30.97 g/mol × 1 = 30.97 g/mol
- Oxygen (O): 16.00 g/mol × 4 = 64.00 g/mol
Thus, the molar mass of phosphoric acid is approximately 97.99 g/mol. This information is vital in industrial processes and laboratory experiments where accurate measurements are crucial.
Environmental Impact of Phosphoric Acid
The widespread use of phosphoric , based on its formula, raises concerns about its environmental impact. In agricultural use, excessive application of phosphate-based fertilizers can lead to water pollution through runoff, resulting in harmful algae blooms. Understanding the chemistry behind H₃PO₄ can help industries manage its use responsibly.
Safety Precautions When Handling Phosphoric Acid
While phosphoric acid is highly useful, it can also be hazardous if not handled properly. Its acidic nature, reflected in the formula, means it can cause burns or irritation to the skin, eyes, and respiratory system. Always wear appropriate safety gear, such as gloves and goggles, when working with phosphoric acid in any form.
Why Understanding the Formula for Phosphoric Acid is Essential
Understanding the formula for phosphoric is critical for anyone working in chemistry, manufacturing, or industrial sectors. The H₃PO₄ structure gives insights into how phosphoric acid behaves in reactions and how it can be safely and effectively used. From agriculture to food processing, this formula serves as the foundation for countless applications.
Final Thoughts on the Formula for Phosphoric Acid
Phosphoric, with the formula H₃PO₄, is an indispensable compound in various industries. Its applications range from agriculture to food production, metal treatment, and pharmaceuticals. Knowing the formula for phosphoric not only helps in understanding its properties but also in leveraging it for industrial purposes.
