Blog
What is Acetic Acid: A Comprehensive Guide to its Properties
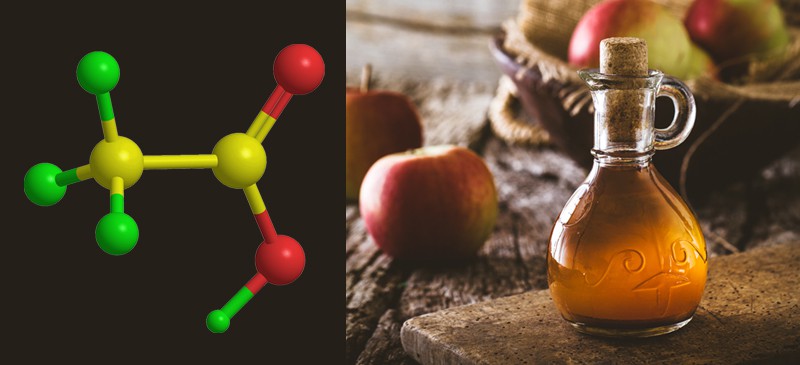
Acetic acid is a colorless liquid organic compound with a pungent smell and sour taste. Known as the main component of vinegar, it plays a significant role in both the chemical industry and everyday life. But what is acetic acid exactly? Its chemical formula is CH₃COOH, and it belongs to the family of carboxylic acids. This versatile substance is widely used in food production, manufacturing, and even medical applications.
Acetic acid has been around for centuries, first discovered in vinegar, and is now produced on a large scale for various uses. In this article, we’ll explore its definition, properties, production process, and uses.
The Chemical Structure of Acetic Acid
To understand what acetic acid is, we need to look at its chemical structure. Acetic acid contains two carbon atoms, four hydrogen atoms, and two oxygen atoms, forming the formula CH₃COOH. It is classified as a weak acid because it only partially ionizes in water. However, its strength is enough to give vinegar its sour taste and to serve as a significant chemical reagent in the industry.
How is Acetic Acid Produced?
Acetic acid can be produced both naturally and synthetically.
Natural Production of Acetic Acid
Acetic acid naturally forms through the fermentation process, where ethanol is converted into acetic acid by acetic acid bacteria. This process is commonly used to make vinegar. When exposed to oxygen, ethanol found in alcoholic beverages is fermented into acetic acid, resulting in the tangy flavor of vinegar.
Synthetic Production of Acetic Acid
Industrially, acetic acid is produced using several methods. The most common one is the carbonylation of methanol, a process where methanol and carbon monoxide react under pressure and heat to form acetic acid. This method accounts for the majority of acetic acid produced globally and is used in large-scale manufacturing processes.
What is Acetic Acid Used For?
Acetic has a wide range of applications across various industries. Let’s take a closer look at some of the most common uses of acetic .
Food Industry
Acetic is a key ingredient in vinegar, which is widely used as a food preservative and flavoring agent. It helps in preventing the growth of harmful bacteria in pickles, sauces, and dressings. Besides, acetic is used in food production to maintain the proper acidity level in processed foods.
Chemical Manufacturing
In the chemical industry, acetic is used as a precursor to many compounds. It is essential in producing acetic anhydride, a chemical used to manufacture cellulose acetate, which is a material found in photographic films, textiles, and plastics. Acetic is also used in the production of synthetic fibers, adhesives, and solvents.
Medical and Pharmaceutical Applications
Acetic is found in various medical applications, including its use as an antiseptic. It is effective in treating ear infections and preventing bacterial growth in wounds. Additionally, it is used in the production of pharmaceuticals, such as aspirin, where acetic plays a critical role in the chemical synthesis process.
Household Cleaning Products
Due to its antibacterial properties, acetic is commonly found in household cleaning products. It helps to remove grime, limescale, and mold from surfaces, making it a popular ingredient in eco-friendly cleaning solutions.
What are the Properties of Acetic Acid?
Physical Properties
Acetic is a colorless liquid with a sharp, vinegar-like smell. Its boiling point is 118°C, and it has a melting point of 16.6°C. It is miscible with water, meaning it can mix in any proportion without separating. Acetic has a relatively low pH, which makes it an acidic substance that can react with bases and metals.
Chemical Properties
As a weak acid, acetic only partially dissociates in water. Its chemical reactivity allows it to engage in a variety of reactions, such as esterification and the formation of acetates, which are widely used in industrial processes. Acetic is also flammable in concentrated forms, requiring careful handling in production settings.
Acetic Acid in Everyday Life
Vinegar and Cooking
One of the most well-known uses of acetic is in vinegar. Used for cooking, pickling, and preserving, vinegar has been an essential household staple for centuries. The acetic content in vinegar typically ranges from 4% to 8%, making it strong enough to preserve food without overpowering the flavor.
Cleaning Agent
Acetic is also used as an eco-friendly cleaning agent in households. It effectively removes tough stains and acts as a natural disinfectant. People often use vinegar (acetic) to clean windows, kitchen surfaces, and even laundry.
Health and Wellness
In small, diluted quantities, acetic has health benefits. While not a cure-all, it has been a part of traditional remedies for centuries.
The Environmental Impact of Acetic Acid
Acetic is biodegradable and breaks down easily in the environment. While large concentrations may pose a risk to aquatic life, acetic generally considered environmentally safe in moderate amounts. Industrial plants ensure that they meet environmental standards to reduce any potential harm caused by acetic emissions.
Conclusion
Acetic is an incredibly versatile and essential substance in both our daily lives and industrial processes. From its role in food production and cleaning to its applications in chemical manufacturing and medicine, acetic acid’s importance cannot be overstated. Understanding what acetic is and how it is produced helps us appreciate its wide range of uses and benefits.
