Blog
What is a pH of Acetic Acid? Learn More About Acidity Levels
Acetic acid is a common organic compound found in vinegar, and one of its essential properties is its pH level. What is a pH of acetic ? It generally falls around 2.4, making it a weak acid. Understanding the pH of acetic acid is crucial in various fields, such as chemistry, food preservation, and industry, as it helps determine its acidity and effectiveness in different applications.
In this article, we’ll dive deep into the concept of pH, how the pH of acetic compares to other acids, and why it matters in both practical and industrial contexts.
What is pH and Why Does It Matter?
Before answering the question, “What is a pH of acetic ?” let’s explore what pH means. pH is a measure of how acidic or basic a solution is, on a scale of 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while those above 7 are alkaline (or basic).
- A lower pH value (closer to 0) means the solution is highly acidic.
- A higher pH value (closer to 14) indicates a strong alkaline substance.
Acids like acetic acid dissociate into ions when dissolved in water, contributing hydrogen ions (H+), which is what makes them acidic. Acetic acid, although classified as a weak acid, plays a vital role in many everyday uses.
What is a pH of Acetic Acid Compared to Other Acids?
What is a pH of acetic compared to stronger acids like hydrochloric acid or sulfuric acid? The pH of acetic acid, which is around 2.4 to 2.5 depending on its concentration, is much higher (less acidic) than strong acids like hydrochloric acid, which has a pH close to 1.
Here’s a comparison of the pH of acetic with other common acids:
- Hydrochloric Acid (HCl): pH 1
- Sulfuric Acid (H2SO4): pH 0.3
- Lemon Juice (Citric Acid): pH 2
- Acetic Acid (Vinegar): pH 2.4 to 2.5
As you can see, acetic acid is relatively mild compared to these stronger acids, but it is still acidic enough for household uses like cleaning or food preservation.
Factors Affecting the pH of Acetic Acid
The pH of acetic can vary slightly depending on the concentration of the solution. Higher concentrations of acetic will lower the pH, making it more acidic, while diluted solutions will have a higher (less acidic) pH.
- Concentration: Pure acetic acid, also known as glacial acetic, is much more acidic (pH around 2.4) compared to diluted vinegar solutions, which may have a pH closer to 3.
- Temperature: pH can be temperature-dependent. A higher temperature can slightly lower the pH of acetic acid, as more acetic molecules dissociate into ions.
Understanding these factors is crucial in various industries, especially food manufacturing, where controlling the acidity level ensures product safety and effectiveness.
Why Understanding the pH of Acetic Acid is Important
Knowing what is a pH of acetic is important for several reasons:
- Food Preservation: Acetic acid is widely used in the food industry for preserving pickles and other foods. Its acidity prevents the growth of harmful bacteria and fungi.
- Household Uses: Vinegar, a dilute solution of acetic acid, is used for cleaning and disinfecting because its acidity helps break down grime and kills bacteria.
- Medical Applications: Acetic is sometimes used in medical treatments, especially for treating ear infections and burns, where its pH plays a vital role in its effectiveness.
- Chemical Industry: Acetic acid is used as a chemical reagent and in the production of synthetic fibers, paints, and plastics. Knowing its pH ensures the right conditions for these chemical reactions.
In short, understanding the pH of acetic helps maximize its effectiveness and safety in multiple areas.
How to Measure the pH of Acetic Acid
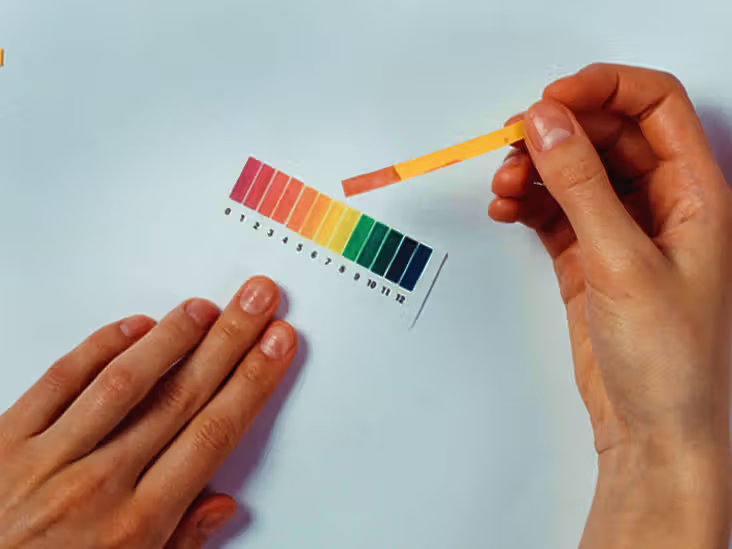
Measuring the pH of acetic is relatively simple and can be done using pH meters or pH indicator strips. These tools provide an accurate reading of how acidic or basic a solution is.
- pH Meter: A pH meter provides a precise measurement by dipping the electrode into the acetic solution. The meter displays the pH on a digital screen.
- pH Strips: These are paper strips that change color based on the acidity of the solution. Comparing the strip’s color to a pH chart can give an approximate pH level.
Both methods are widely used in laboratories and even at home to check the acidity of substances like vinegar or other acetic acid solutions.
What is a Safe pH of Acetic Acid for Everyday Use?
What is a pH of acetic that is safe for daily use? Vinegar, which is 4-6% acetic acid, is considered safe for consumption, cleaning, and other household tasks. The pH of vinegar typically ranges between 2.5 to 3.5, which is mild enough to be safe, but still acidic enough to kill bacteria and clean surfaces.
If you’re using concentrated acetic (such as in industrial applications), handling it with care is essential because higher concentrations have lower pH levels and are more corrosive. Gloves, goggles, and proper ventilation are recommended for handling pure or highly concentrated acetic acid.
Practical Applications of Acetic Acid Based on pH
The pH of acetic influences its practical applications in daily life and industries.
- Food Industry: Its acidity helps in pickling and preserving food items.
- Cleaning Agent: Vinegar is a popular cleaning agent due to its ability to cut through grease and kill bacteria.
- Agriculture: Acetic acid is sometimes used as an herbicide because its acidity damages plant cells.
Knowing how the pH of acetic behaves helps in determining its effectiveness for specific applications.
Conclusion: What is a pH of Acetic Acid and Why You Should Know
In summary, what is a pH of acid? It is typically around 2.4 in pure form but can vary based on concentration and temperature. Understanding the pH of acid is important in food preservation, cleaning, medicine, and more. From home use to industrial applications, this weak acid plays a significant role in maintaining safety and efficiency in various fields.
