Blog
Sodium Carbonate Structure: Understanding its Composition
The sodium carbonate structure is essential in many industries, from glass manufacturing to water treatment. Sodium carbonate, commonly known as soda ash or washing soda, is an inorganic compound with a chemical formula of Na₂CO₃. Its crystalline structure plays a crucial role in its chemical properties and various applications. In this article, we will dive into the structure of sodium carbonate, its properties, and how it benefits different industries.
What is Sodium Carbonate?
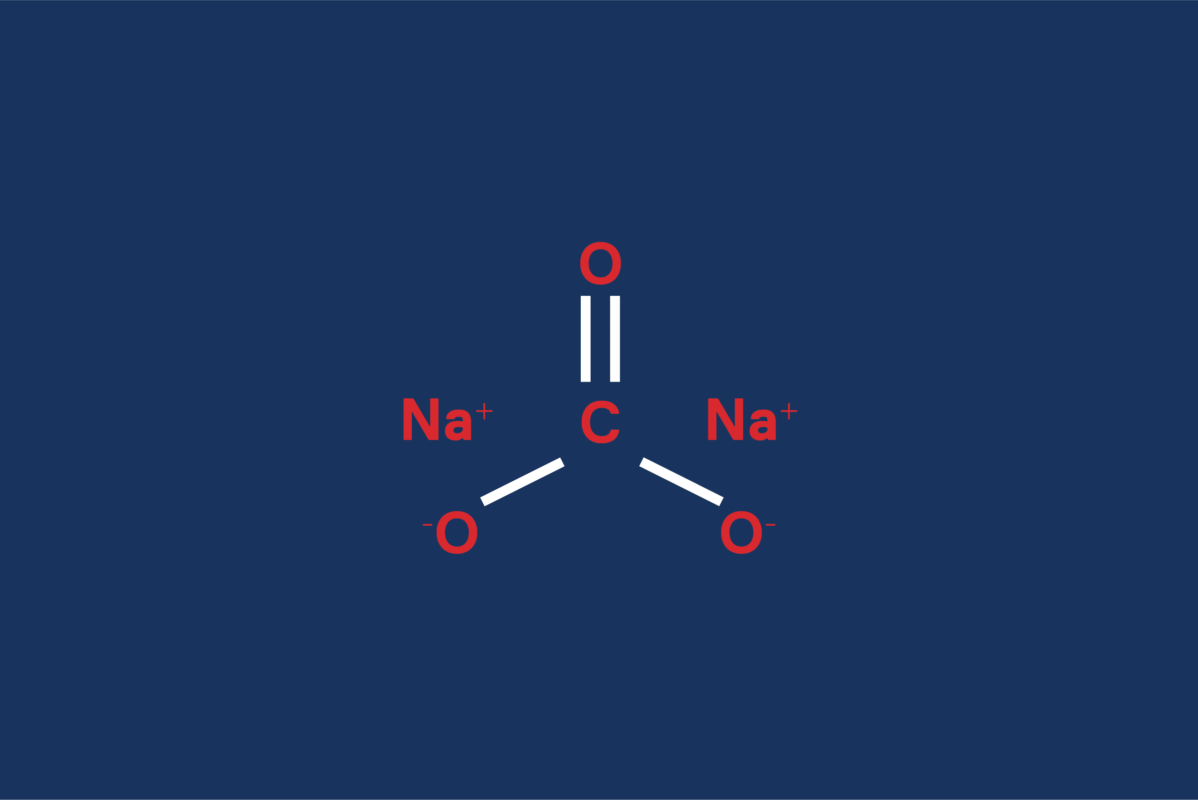
Sodium carbonate consists of sodium and carbonate ions. It dissolves easily in water and serves various applications because of its alkaline nature. The structure of sodium carbonate consists of two sodium ions (Na⁺) and one carbonate ion (CO₃²⁻). This combination gives the compound its unique properties, making it valuable for both domestic and industrial use.
Importance of Sodium Carbonate Structure
Understanding the sodium carbonate structure is critical for comprehending its chemical behavior. The structure is primarily responsible for the compound’s ability to neutralize acids and participate in chemical reactions, making it an essential substance in industrial processes like glass manufacturing, soap production, and water softening.
Chemical Structure of Sodium Carbonate
The chemical structure of sodium carbonate is simple but highly effective. It contains two sodium ions (Na⁺) and one carbonate ion (CO₃²⁻), which are arranged in a crystalline form. This crystalline structure allows sodium carbonate to exist in different hydrated forms, such as monohydrate (Na₂CO₃·H₂O) and decahydrate (Na₂CO₃·10H₂O).
Sodium Ions in Sodium Carbonate Structure
The sodium ions in the sodium carbonate structure play a key role in maintaining the stability of the compound. These ions are positively charged and are attracted to the negatively charged carbonate ions, forming a strong ionic bond. This bond is what gives sodium carbonate its stability and reactivity.
Carbonate Ion in Sodium Carbonate Structure
The carbonate ion (CO₃²⁻) in the sodium carbonate structure contains one carbon atom and three oxygen atoms. The atoms bond in a trigonal planar arrangement, creating the ion’s flat, symmetrical shape. The carbonate ion is responsible for the compound’s alkalinity, making sodium carbonate a popular choice for neutralizing acids in various industrial processes.
Hydrated Forms of Sodium Carbonate
Sodium can exist in several hydrated forms, meaning it can incorporate water molecules into its structure. The most common forms are monohydrate and decahydrate.
- Monohydrate (Na₂CO₃·H₂O): This form contains one molecule of water per sodium carbonate molecule.
- Decahydrate (Na₂CO₃·10H₂O): In this form, sodium carbonate binds with ten water molecules. This form is commonly found in nature as natron.
Each hydrated form of sodium carbonate has its own unique properties, making them suitable for different applications.
Applications of Sodium Carbonate
The sodium structure enables it to perform a wide range of functions. Here are some of the most common applications:
Glass Manufacturing
Sodium carbonate is a vital component in glass production. Its structure allows it to lower the melting point of silica, making the glass-forming process more efficient. The sodium structure helps improve the quality and durability of the glass produced.
Water Softening
The sodium structure makes it an effective water softener. It binds to calcium and magnesium ions in hard water, removing them and making the water softer. This prevents scale buildup in pipes and improves the effectiveness of soaps and detergents.
Cleaning Agent
Due to its alkaline properties, sodium carbonate is used in cleaning products. The sodium carbonate allows it to break down grease and remove stains, making it a popular ingredient in household cleaning agents.
pH Regulation
The ability of the sodium structure to neutralize acids makes it valuable in various chemical processes. It is commonly used to regulate pH levels in industries like food processing, pharmaceuticals, and water treatment.
Sodium Carbonate Structure in Nature
Sodium carbonate occurs naturally in mineral deposits, such as trona and natron. The naturally occurring sodium carbonate is identical to the synthetic version used in industrial applications. Sodium carbonate naturally occurs in dry lake beds, and industries extract it for use in a variety of products.
Conclusion
Understanding the sodium structure is essential for appreciating its versatility and wide range of applications. From glass manufacturing to water treatment, the structure of sodium plays a significant role in its usefulness across various industries. Its simple yet effective composition of sodium and carbonate ions allows it to perform important functions that benefit both consumers and businesses alike.
