Blog
Molar Mass of Acetic Acid
The molar mass of acetic acid is a fundamental concept in chemistry that helps in understanding various chemical reactions and processes. Acetic acid, known scientifically as CH₃COOH, is a common organic compound found in vinegar. It is crucial in fields such as stoichiometry, where it is used to determine the quantities of substances involved in chemical reactions. In this guide, we will break down the calculation to explore its significance, and provide practical examples.
Understanding the Molar Mass of Acetic Acid
What is Molar Mass?
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). To calculate the mass of acetic acid (CH₃COOH), sum the atomic masses of all the atoms in the molecule. Add the atomic masses of carbon (C), hydrogen (H), and oxygen (O) found in acetic acid.
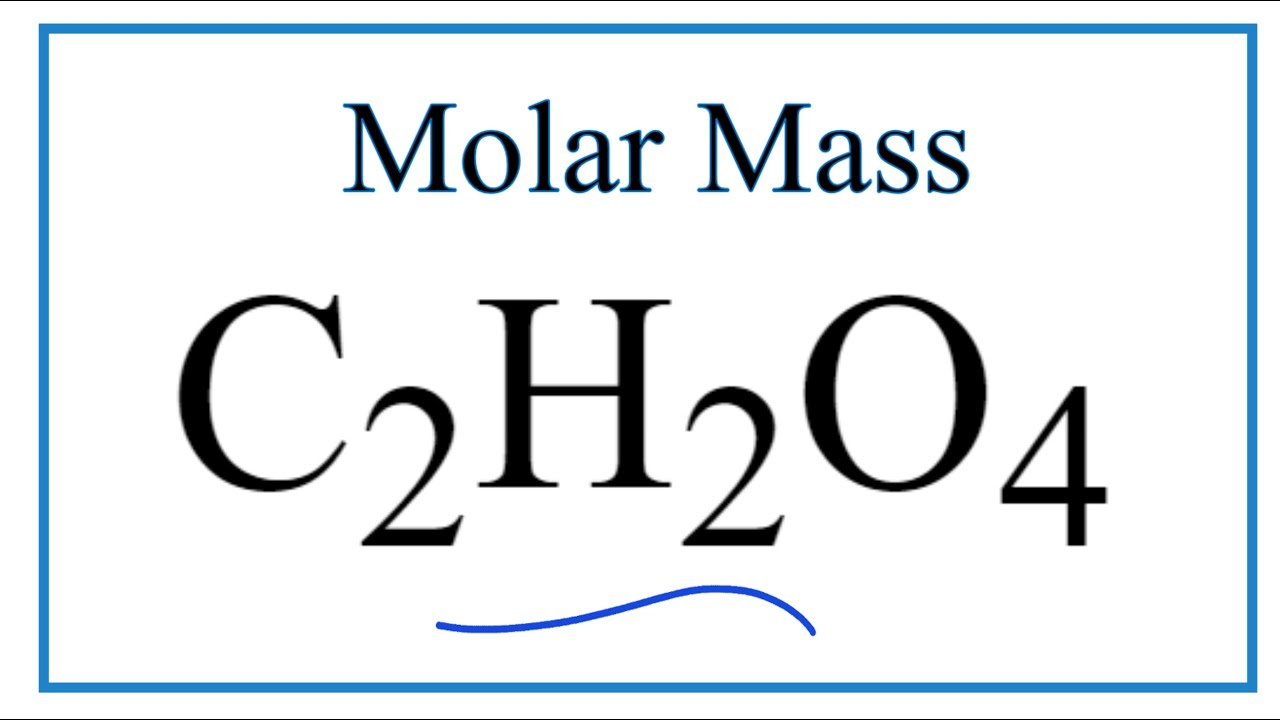
How to Calculate the Molar Mass of Acetic Acid
Calculating the molar mass of acetic acid involves simple arithmetic. The molecular formula of acetic acid is CH₃COOH, which consists of:
- 2 Carbon atoms (C)
- 4 Hydrogen atoms (H)
- 2 Oxygen atoms (O)
Using the atomic masses:
- Carbon (C) = 12.01 g/mol
- Hydrogen (H) = 1.008 g/mol
- Oxygen (O) = 16.00 g/mol
The molar mass of acetic acid is calculated as follows:Molar Mass of Acetic Acid=(2×12.01)+(4×1.008)+(2×16.00)=24.02+4.032+32.00=60.052 g/mol\text{Molar Mass of Acetic Acid} = (2 \times 12.01) + (4 \times 1.008) + (2 \times 16.00) = 24.02 + 4.032 + 32.00 = 60.052 \, \text{g/mol}Molar Mass of Acetic Acid=(2×12.01)+(4×1.008)+(2×16.00)=24.02+4.032+32.00=60.052g/mol
Thus, the molar mass of acetic acid is 60.052 g/mol.
Importance of Knowing the Molar Mass of Acetic Acid
Applications in Chemistry
Understanding it is essential in various chemical applications. For instance, it is used to calculate the number of moles in a given mass of acetic acid, which is a crucial step in stoichiometric calculations. These calculations help chemists determine the proportions of reactants and products in chemical reactions, ensuring accurate and efficient experimentation.
Role in Industry and Everyday Life
Acetic acid serves as a preservative and flavoring agent in the food industry. It plays a crucial role in industrial applications, where precise concentrations are necessary. Additionally, manufacturers use acetic acid to produce various chemicals, such as acetate esters, which are essential for creating plastics, textiles, and other materials.
Practical Examples of Using Molar Mass of Acetic Acid
Example 1: Determining the Number of Moles
Imagine you have 30 grams of acetic acid and you need to determine how many moles that corresponds to. Using the mass of acetic acid (60.052 g/mol), you can easily calculate it:Number of Moles=Mass of Acetic AcidMolar Mass of Acetic Acid=30 g60.052 g/mol≈0.50 moles\text{Number of Moles} = \frac{\text{Mass of Acetic }}{\text{Molar Mass of Acetic Acid}} = \frac{30 \, \text{g}}{60.052 \, \text{g/mol}} \approx 0.50 \, \text{moles}Number of Moles=Molar Mass of Acetic AcidMass of Acid=60.052g/mol30g≈0.50moles
Example 2: Preparing a Solution
Suppose you need to prepare a 1 M (molar) solution of acetic acid. This means you need 1 mole of acetic acid per liter of solution. Knowing the molar of acetic acid allows you to measure the correct amount of the substance. For a 1-liter solution, you would need 60.052 grams of acetic acid.
Significance of Acetic Acid in Various Fields
Acetic Acid in Food and Beverage
Acetic acid’s role in food preservation and flavor enhancement is well known. Its antimicrobial properties make it an essential ingredient in pickling, while its distinct sour taste contributes to the flavor profile of many dishes.
Acetic Acid in Chemical Synthesis
In chemical synthesis, chemists use acetic acid as a reagent to produce other chemicals. The molar mass of acetic acid plays a critical role in calculating the necessary quantities for reactions, ensuring efficient production of the desired products.
Tips for Remembering the Molar Mass of Acetic Acid
Mnemonic Devices
To remember, you can use mnemonic devices such as “Two Cats Have Four Orange Oranges” to recall the number of atoms in the molecule: 2 C, 4 H, and 2 O. Multiplying the atomic masses accordingly gives you the molar mass.
Practice Problems
Regularly practicing the calculation of molar masses for various compounds, including acetic acid, will reinforce your understanding and help you remember the values more easily.
Conclusion: Mastering the Molar Mass of Acetic Acid
Understanding a crucial skill for anyone studying or working in chemistry. Whether you’re a student preparing for exams, a researcher conducting experiments, or a professional in the chemical industry, mastering this concept will significantly enhance your ability to work with chemical substances accurately.
FAQS
How do you find the molar mass of acetic acid?
To find the molar mass of acetic, you need to sum the atomic masses of all the atoms present in its molecular formula, which is CH₃COOH. The calculation is as follows:
- Carbon (C): 2 atoms × 12.01 g/mol = 24.02 g/mol
- Hydrogen (H): 4 atoms × 1.008 g/mol = 4.032 g/mol
- Oxygen (O): 2 atoms × 16.00 g/mol = 32.00 g/mol
Adding these values together gives you the molar mass of acetic :
[\text{Molar Mass of Acetic Acid} = 24.02 + 4.032 + 32.00 = 60.052 \, \text{g/mol}
]
How to find the molecular mass of CH₃COOH?
The molecular mass of CH₃COOH (acetic acid) is found by adding the atomic masses of the constituent atoms:
- Carbon (C): 2 atoms × 12.01 g/mol = 24.02 g/mol
- Hydrogen (H): 4 atoms × 1.008 g/mol = 4.032 g/mol
- Oxygen (O): 2 atoms × 16.00 g/mol = 32.00 g/mol
The total molecular mass is:
[\text{Molecular Mass of CH₃COOH} = 24.02 + 4.032 + 32.00 = 60.052 \, \text{g/mol}
]
What is the molar mass of acetic acid g/ml?
It is typically expressed in grams per mole (g/mol), not grams per milliliter (g/ml). The molar mass of acid is 60.052 g/mol. If you need to convert this to grams per milliliter, you would need the density of acetic acid, which is approximately 1.049 g/ml for pure acetic acid at room temperature. However, molar mass itself remains as g/mol.
What is the molar mass of pure acetic acid?
The molar of pure acetic acid is 60.052 g/mol. This value is calculated based on its molecular formula, CH₃COOH, by summing the atomic masses of all the atoms in the molecule.

